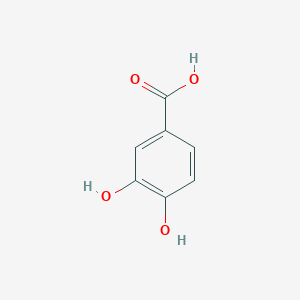
3,4-dihydroxybenzoic acid
| Common Name: |
3,4-dihydroxybenzoic acid |
| IUPAC Name: |
3,4-dihydroxybenzoic acid |
| Molecular Formula: |
C7H6O4 |
| SMILES: |
C1=CC(=C(C=C1C(=O)O)O)O |
| Inchi: |
1S/C7H6O4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H,(H,10,11) |
| Inchi Key: |
YQUVCSBJEUQKSH-UHFFFAOYSA-N |
| Cas No: |
99-50-3 |
| Name |
Value |
| Lipinski Violations |
0 |
| Ghose Violations |
3 |
| Veber Violations |
0 |
| Egan Violations |
0 |
| Muegge Violations |
1 |
| Name |
Value |
| Molecular Weight (g/mol) |
154.12 |
| Mass (g/mol) |
154.027 |
| Molar Refractivity |
37.45 |
| Net Charge |
-1 |
| HBD |
3 |
| HBA |
4 |
| Rt Bonds |
1 |
| Rings |
1 |
| TPSA |
77.76 |
| Hetero Atoms |
4 |
| Heavy Atoms |
11 |
| Aromatic Heavy Atoms |
6 |
| Melting Point (°C) |
221 |
| Boiling Point (°C@760.00mm Hg) |
170.00 to 171.00 |
| Vapor Pressure (mmHg@25.00 °C) |
|
| Vapor Density (Air =1) |
|
| Fraction Csp3 |
0.00 |
| LogP |
0.796 |
| iLOGP |
0.66 |
| XLOGP3 |
1.15 |
| WLOGP |
0.80 |
| MLOGP |
0.40 |
| ESOL Log S |
-1.86 |
| ESOL Solubility (mg/ml) |
2.14 |
| ESOL Solubility (mol/l) |
0.014 |
| ESOL Class: esol_class |
Very soluble |
| Ali Log S |
-2.38 |
| Ali Solubility (mg/ml) |
0.65 |
| Ali Solubility (mol/l) |
0 |
| Ali Class |
Soluble |
| Silicos-IT LogSw |
-0.60 |
| Silicos-IT Solubility (mg/ml) |
38.3 |
| Silicos-IT Solubility (mol/l) |
0.25 |
| Silicos-IT Class |
Soluble |
| Name |
Value |
| GI Absorption |
High |
| BBB Permeable |
0 |
| PgP Substrate |
0 |
| Log Kp (cm/s) |
-6.42 |
| Bioavailability Score |
0.56 |
| Caco2 |
0 |
| Human Intestinal Absorption |
1 |
| Plasm Protein Binding |
0.717 |
| CYP1A2 Inhibitor |
0 |
| CYP2C19 Inhibitor |
0 |
| CYP2C9 Inhibitor |
0 |
| CYP2D6 inhibitor |
0 |
| CYP3A4 inhibitor |
1 |
| Ames mutagenesis |
0 |
| Acute Oral Toxicity |
1.111 |
| Carcinogenicity (Binary) |
0 |
| Carcinogenicity (Trinary) |
Non-required |
| Eye Irritation |
1 |
| Hepatotoxicity |
0 |
| Androgen Receptor Binding |
0 |
| Aromatase Binding |
0 |
| Estrogen Receptor Binding |
0 |
| Glucocorticoid Receptor Binding |
0 |
| Thyroid Receptor Binding |
0 |
| BRCP inhibitor |
0 |
| BSEP inhibitor |
0 |
| OATP1B1 inhibitor |
1 |
| OATP1B3 inhibitor |
1 |
| OATP2B1 inhibitor |
0 |
| OCT1 inhibitor |
0 |
| OCT2 inhibitor |
0 |