OlfactionBase:
A repository of Odors, Odorants, Olfactory Receptors and related information
- Home
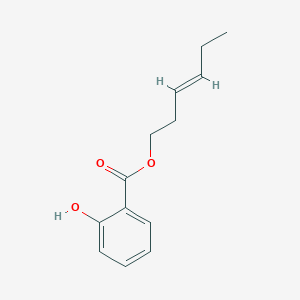
- Odorants
Sharma A, Saha BK, Kumar R, Varadwaj PK. OlfactionBase: a repository to explore odors, odorants,
olfactory receptors and odorant-receptor interactions. Nucleic Acids Res. 2022 Jan 7;50(D1):D678-D686.
doi: 10.1093/nar/gkab763.